![[Previous Page]](btn_prev.gif)
![[Index]](btn_indx.gif)
![[Next Page]](btn_next.gif)

![[Previous Page]](btn_prev.gif)
![[Index]](btn_indx.gif)
![[Next Page]](btn_next.gif)
[3] Ozone holes aren't like doughnut holes; they're not empty spaces in the sky. Ozone holes are much like the worn-out places in an old sock or sweater: there are still threads covering the worn-out area, but the fabric can be so thin you can see right through it.
Ozone in the stratosphere, a layer of the atmosphere nine to 31 miles above the Earth, serves as a protective shield, filtering out harmful sun rays, including a type of sunlight called ultraviolet B. Exposure to ultraviolet B has been linked to development of cataracts (eye damage) and skin cancer.
In the mid 1970s, scientists suggested that chlorofluorocarbons (CFCs) could destroy stratospheric ozone. CFCs were widely used then as aerosol propellants in consumer products such as hairsprays and deodorants, and for many uses in industry. Because of concern about the possible effects of CFCs on the ozone layer, in 1978 the U.S. government banned CFCs as propellants in aerosol cans.
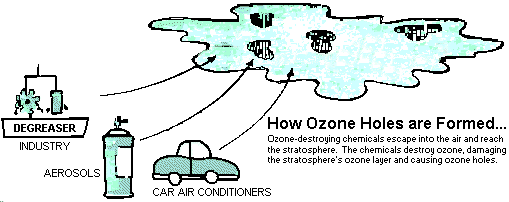

Evidence that the ozone layer is dwindling led 93 nations, including the major industrialized nations, to agree to cooperate in reducing production and use of chemicals that destroy the ozone layer. As it became clear that the ozone layer was thinning even more quickly than first thought, the agreement was revised to speed up the phase-out of ozone-destroying chemicals.
Unfortunately, it will be a long time before we see the ozone layer repaired. Because of the ozone-destroying chemicals already in the stratosphere and those that will arrive within the next few years, ozone destruction will likely continue for another twenty years.
The 1990 Clean Air Act sets a schedule for ending production of chemicals that destroy stratospheric ozone. Chemicals that cause the most damage will be phased out first. The phase-out schedule can be speeded up if an earlier end to production of ozone-destroying substances is needed to protect the ozone layer. The table on this page on Ozone-destroying chemicals includes "speeded-up" phase-out dates which were proposed by EPA in early 1993.
| Ozone-destroying chemicals | ||
|---|---|---|
| Name | Use | When U. S. production ends* |
| CFCs (chlorofluorocarbons) | solvents, aerosol sprays (most spray can uses banned in 1970s) foaming agents in plastic manufacture | January 1, 1996 |
| Halons | fire extinguishers | January 1, 1994 |
| Carbon tetrachloride | solvents, chemical manufacture; carbon tetrachloride causes cancer in animals | January 1, 1996 |
| Methyl chloroform (1,1,1-trichloroethene) | very widely-used solvent; in many workplace and consumer solvents, including auto repair and maintenance products | January 1, 1996 |
| HCFCs (hydro CFCs) | CFC substitutes, chemicals slightly different from CFCs | January 1, 2003** |
*The 1990 Clean Air Act includes a schedule for ending United States production of ozone-destroying chemicals and provisions for speeding up the phase out schedule if that is necessary. The dates in this table are "speeded-up" dates, proposed by EPA in early 1993.
**Production of the HCFC with the most severe ozone destroying effects will end by January 1, 2003. Production of the rest of the HCFCs will end by January 1, 2030.
CFCs, Halons, HCFCs (hydrochlorofluorocarbons)[4] and other ozone-destroying chemicals were listed by Congress in the 1990 Clean Air Act and must be phased out. The Act also lets EPA list other chemicals that destroy ozone.
[4] HCFCs and Halons are chemicals much like CFCs. HCFCs may be somewhat less harmful to the ozone layer than are CFCs.
EPA issues allowances to control manufacture of chemicals being phased out. Companies can also sell unused allowances to companies still making the chemicals or can use the allowances, within certain limits, to make a different, less ozone-destroying chemical on the phase-out list.
In addition to requiring the phasing out of production of ozone-destroying chemicals, the Clean Air Act takes other steps to protect the ozone layer. The law requires recycling of CFCs and labeling of products containing ozone-destroying chemicals. The 1990 Clean Air Act also encourages the development of "ozone-friendly" substitutes for ozone-destroying chemicals.
CFCs from car air conditioners are the biggest single source of ozone-destroying chemicals. By the end of 1993, all car air conditioner systems must be serviced using equipment that recycles CFCs and prevents their release into the air. Larger auto service shops were required to start using this special equipment in January 1992. Only specially-trained and certified repair persons will be allowed to buy the small cans of CFCs used in servicing auto air conditioners.
As CFCs and related chemicals are phased out, appliances and industrial processes that now use the chemicals will change. For example, industrial and home refrigerators will be changed to use refrigerants that don't destroy ozone. In the meantime, refrigerator servicing and disposal will have to be done in ways that don't release CFCs. Methyl chloroform, also called l,l,l-trichloroethane, which will be phased out by 1996, is a very widely-used solvent found in products such as automobile brake cleaners (often sold as aerosol sprays) and spot removers used to take greasy stains off fabrics. Replacing methyl chloroform in workplace and consumer products will lead to changes in many products and processes.
As substitutes are developed for ozone-destroying substances, before the chemicals can be produced and sold, EPA must determine that the replacements will be safe for health and the environment.
Consumer products containing CFCs and other ozone-destroying chemicals will have to be reformulated; these are discussed in the following section on Consumer products.
![[Previous Page]](btn_prev.gif)
![[Index]](btn_indx.gif)
![[Next Page]](btn_next.gif)